New EU regulations enforcing a 9-month limit on the efficacy of the COVID-19 vaccine - Netherlands News Live

New European Union Regulations for Medical Devices and In vitro Devices — Are You Ready to Thrive in the New Landscape? - CQ fluency

ONLINE EXCLUSIVE: Revised EU regulations light the way to more stringent requirements for light sources | LEDs Magazine

Other - New EU Regulations on veterinary medicines - Teagasc | Agriculture and Food Development Authority

Case Study: Complying with New EU Medical Device Regulations on Unique Device Identifiers - SL Controls

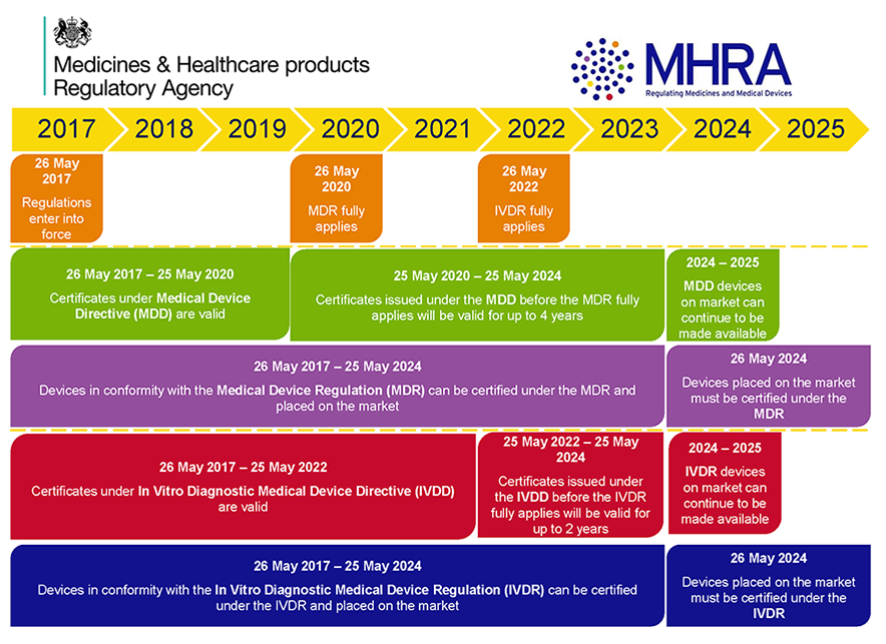
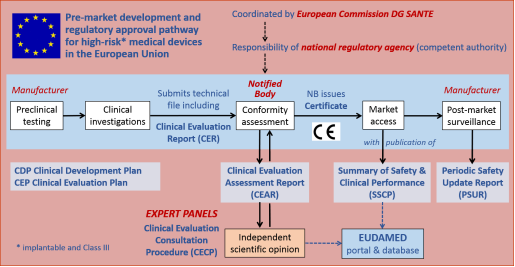
New EU regulations on medical devices: What changes from May 26, 2020? - PreScouter - Custom Intelligence from a Global Network of Experts